Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.




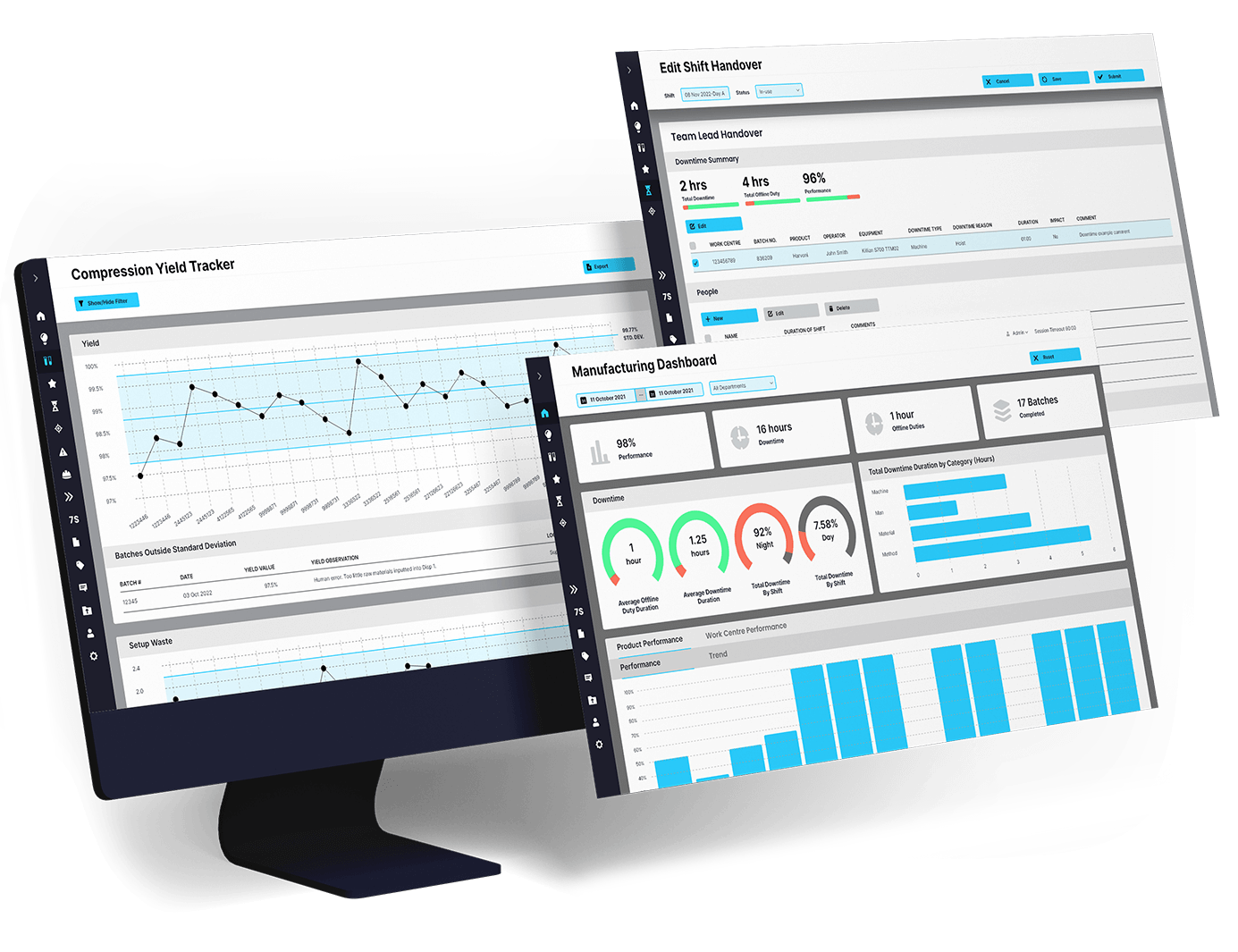
Pharmaceutical manufacturing is an industry of precision and high stakes. Every missed shift handover, siloed data system, or delayed decision can lead to costly downtime, quality risks, or compliance issues.
The good news? These challenges are not roadblocks—they are opportunities to evolve. With digital solutions like EviView’s Digital Daily Management System, pharma manufacturers can turn inefficiencies into actionable insights and build a foundation for sustainable growth.
Seamless shift handovers: Say goodbye to miscommunications. Centralize critical updates so every team starts their shift aligned and ready.
Proactive decision-making: No more relying on outdated reports. Real-time insights empower teams to act quickly, improving quality and reducing downtime.
Effortless safety tracking: Streamline reporting tools that foster a culture of accountability and compliance.
Connected systems and teams: Bring data together from platforms like SAP and Palantir Data Lake for a unified, real-time view of your operations.
These aren’t just hypothetical outcomes—they’re proven results that companies like Merck have already achieved.
Fragmented Data Systems: With operations spread across multiple systems, data silos make it difficult to see the full picture and act quickly.
Inefficient Workflows: Manual processes slow down productivity and leave room for errors, especially during shift handovers.
Compliance Complexities: Safety and regulatory reporting are time-intensive when systems aren’t optimized to handle the workload.
For many manufacturers, these challenges may seem insurmountable. But with the right digital tools, they’re entirely solvable. Just ask Merck.
“EviView’s digital solutions, including their Digital Daily Management System, have been transformative for our operations at Merck. By streamlining shift handovers, centralizing data, and providing real-time integration with systems like SAP and Palantir Data Lake, EviView has enabled us to reduce downtime by 30% and save €66,000 annually.”
— Dr. Nitheen Kaperi Sanyal, Head of Digitalisation and Strategy, Merck
What would reducing downtime by 30% mean for your operations? Or achieving a 300% increase in safety reporting? These results are real—and they can be yours.
📥 Download the full case study here to discover how EviView can empower your operations and help you replicate Merck’s success.
A member of our team would love to introduce you to our platform and answer any questions you may have.
How we can help: