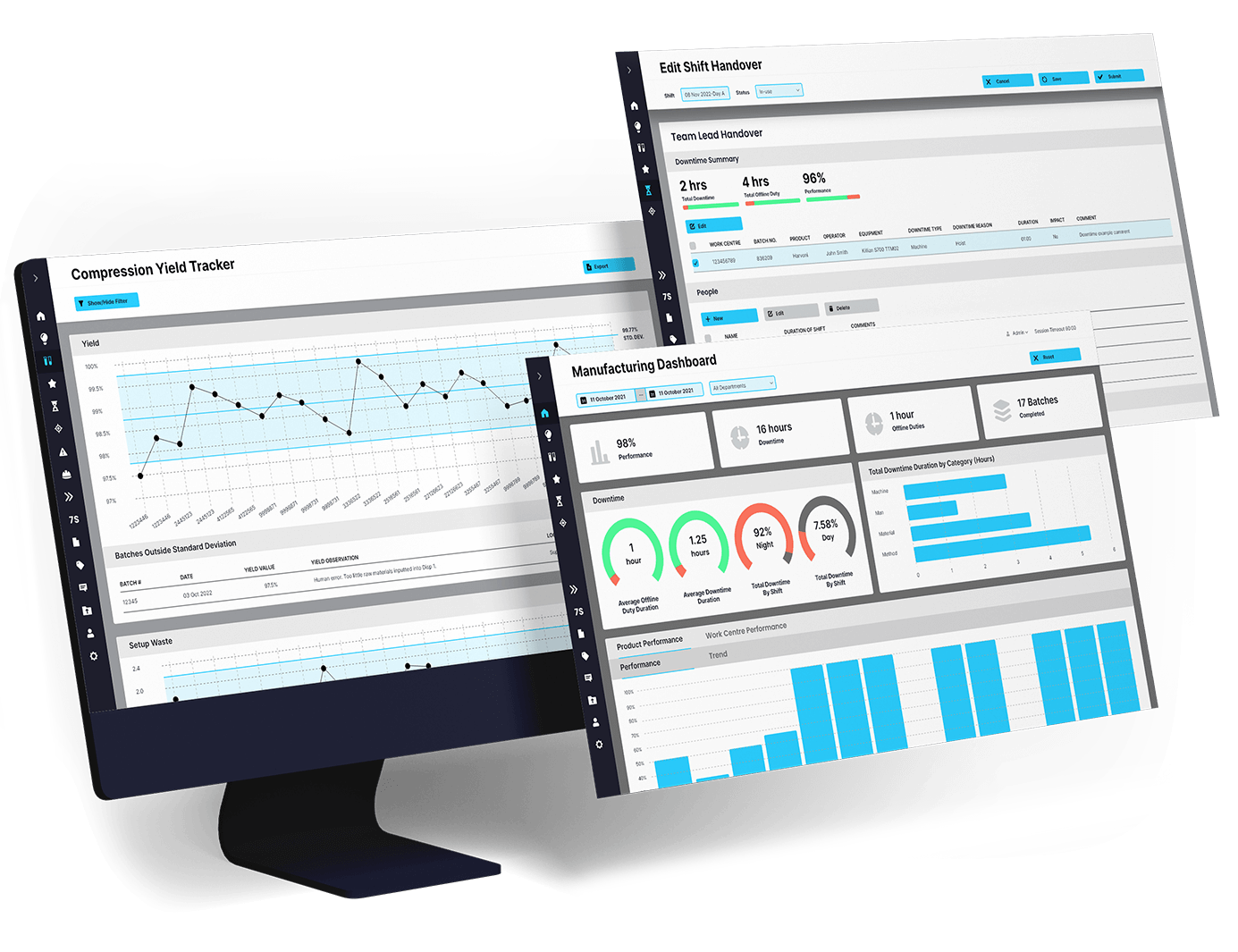
EviView’s digital daily management software offers automated audit management for pharmaceutical regulatory compliance. It is specifically designed to simplify and streamline the audit process. In the highly regulated pharmaceutical industry, audits are critical for ensuring safety, quality, and compliance. EviView automates these tasks, enabling teams to prepare for and execute audits efficiently, with minimal disruption to operations.
By digitizing workflows and integrating real-time data, EviView eliminates the burden of manual audit preparation. Pharmaceutical teams can maintain a state of readiness while ensuring accuracy and reliability in every audit.
Audits can be time-consuming and complex, but EviView simplifies the process with a user-friendly, automated platform.
This includes:
EviView’s software helps pharmaceutical companies save time, improve audit outcomes, and focus on high-value activities.
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.




EviView goes beyond simple audit tracking by offering advanced tools that support continuous improvement and regulatory compliance.
Monitor the progress of ongoing audits and address issues as they arise.
Identify recurring audit findings and implement corrective actions to prevent future non-compliance.

Facilitate teamwork by allowing multiple stakeholders to access and update audit data.
Generate comprehensive audit reports for internal reviews and external inspections.
With these capabilities, pharmaceutical manufacturers can elevate their compliance strategies and maintain operational excellence.
EviView centralizes audit management, creating a single source of truth for all compliance-related data.
Integrate data from production systems to ensure audit records are always up to date.
Maintain a detailed, searchable archive of past audits for quick reference.
Tailor audit tracking and reporting tools to fit your organization’s specific compliance needs.
Protect sensitive compliance data with advanced security measures and role-based access.
By centralizing audit data, EviView empowers pharmaceutical teams to stay ahead of regulatory requirements and ensure consistent compliance.
EviView’s automated tools help pharmaceutical manufacturers optimize every aspect of the audit process.
Identify and address compliance gaps before they become audit findings.

Automate repetitive tasks to minimize human error in audit preparation and execution.

Share audit updates and insights across teams to improve collaboration.

Quickly resolve audit findings with clear, actionable corrective actions.

Leverage audit data to refine processes and enhance overall compliance.

Streamline environmental, health, and safety compliance with tools for incident tracking, audits, and reporting.
EHS
Gain actionable insights into KPIs and process inefficiencies to drive continuous improvement.
MA
Ensure seamless transitions between shifts with tools that track safety, quality, and productivity metrics.
DSh
Since implementing EviView’s system, we’ve seen a dramatic improvement in both safety and efficiency. Incident rates have dropped by 20%, freeing up valuable resources for our safety team. The user-friendly interface and automated reporting have streamlined tasks and improved communication across departments. EviView has been a true game-changer for our commitment to employee well-being and regulatory compliance.
EviView’s digital daily management software offers automated audit management for pharmaceutical regulatory compliance. It is specifically designed to simplify and streamline the audit process. In the highly regulated pharmaceutical industry, audits are critical for ensuring safety, quality, and compliance. EviView automates these tasks, enabling teams to prepare for and execute audits efficiently, with minimal disruption to operations.
By digitizing workflows and integrating real-time data, EviView eliminates the burden of manual audit preparation. Pharmaceutical teams can maintain a state of readiness while ensuring accuracy and reliability in every audit.
Audits can be time-consuming and complex, but EviView simplifies the process with a user-friendly, automated platform.
This includes:
EviView’s software helps pharmaceutical companies save time, improve audit outcomes, and focus on high-value activities.
EviView goes beyond simple audit tracking by offering advanced tools that support continuous improvement and regulatory compliance.
Monitor the progress of ongoing audits and address issues as they arise.
Identify recurring audit findings and implement corrective actions to prevent future non-compliance.

Facilitate teamwork by allowing multiple stakeholders to access and update audit data.
Generate comprehensive audit reports for internal reviews and external inspections.
With these capabilities, pharmaceutical manufacturers can elevate their compliance strategies and maintain operational excellence.
EviView centralizes audit management, creating a single source of truth for all compliance-related data.
Integrate data from production systems to ensure audit records are always up to date.
Maintain a detailed, searchable archive of past audits for quick reference.
Tailor audit tracking and reporting tools to fit your organization’s specific compliance needs.
Protect sensitive compliance data with advanced security measures and role-based access.
By centralizing audit data, EviView empowers pharmaceutical teams to stay ahead of regulatory requirements and ensure consistent compliance.
EviView’s automated tools help pharmaceutical manufacturers optimize every aspect of the audit process.
Identify and address compliance gaps before they become audit findings.

Automate repetitive tasks to minimize human error in audit preparation and execution.

Share audit updates and insights across teams to improve collaboration.

Quickly resolve audit findings with clear, actionable corrective actions.

Leverage audit data to refine processes and enhance overall compliance.

Add Your Heading Text Here
Streamline environmental, health, and safety compliance with tools for incident tracking, audits, and reporting.
EHS
Gain actionable insights into compliance-related KPIs, downtime analysis, and performance tracking.
MA
Ensure seamless transitions between shifts with tools that track safety, quality, and productivity metrics.
DSH
Since implementing EviView’s system, we’ve seen a dramatic improvement in both safety and efficiency. Incident rates have dropped by 20%, freeing up valuable resources for our safety team. The user-friendly interface and automated reporting have streamlined tasks and improved communication across departments. EviView has been a true game-changer for our commitment to employee well-being and regulatory compliance.
EHS Director at Drug Product facility - 750 employees
A member of our team would love to introduce you to our platform and answer any questions you may have.
How we can help: